Hudson B1, Rivera JV2, Rushton W1,2,3
Department of Emergency Medicine, University of Alabama Birmingham
Office of Medical Toxicology, University of Alabama Birmingham
Alabama Poison Information Center, Children’s of Alabama
Introduction
Clenbuterol is a potent, non-selective beta-adrenergic agonist. Exposures in the United States most commonly result from use of adultered illicit substances or abuse for anabolic effects. Treatment is particularly challenging given clenbuterol’s potency and long duration of action. Short-acting beta-adrenergic antagonists, such as esmolol and metoprolol are often utilized; yet reported cases commonly report persistent symptoms despite therapy.
Patient Case
A 45 year-old-male presented to the emergency department (ED) as a transfer for suspected clenbuterol toxicity. The patient reported using 80 mcg of clenbuterol four times per week for weight loss and muscle building. The morning of presentation, he ingested the remainder of his tablets (approximately 100 mcg) in addition to the resin from the bottom of the bottle. Immediately, he experienced severe lightheadedness, diaphoresis, and palpitations.
His initial ED presentation was notable for tachycardia and hypotension (202 beats per minute and 80/43 mmHg, respectively) as well as an acute kidney injury (serum creatinine: 2.47 mg/dL), hypokalemia (2.3 mMol/L), anion gap acidosis, elevated troponin and diffuse ST-segment depressions on electrocardiogram (ECG).
In the ED, he was aggressively treated with aspirin, heparinization, potassium supplementation and intravenous lorazepam. He developed a narrow-complex tachycardia that persisted with rates up to 200 beats per minute with associated hypotension requiring hemodynamic support with a phenylephrine infusion. Due to refractory tachydysrhythmia, esmolol was commenced and rapidly titrated to the maximum infusion rate of 300 mcg/kg/min. The patient was admitted to the intensive care unit (ICU) given the severity of his illness.
The patient went on to develop worsening anxiety and tremulousness proceeding to severe encephalopathy. Toxicology consultation provided recommendations for enteral propranolol 12 hours after an initial presentation. Propranolol 60 mg, followed by another 60 mg dose just under 6 hours later was administered. This resulted in rapid improvement of encephalopathy, hypotension, and tachycardia. The patient received a total of 4 doses of propranolol within a 24-hour period, which allowed for esmolol and phenylephrine infusions to be weaned and subsequently discontinued. The patient was transferred out of the ICU on hospital day 2, and discharged on day 4 with complete resolution of his toxicity. Clenbuterol was noted on anabolic steroid screen, but serum concentrations were not quantified.
Discussion
This case is notable for refractory tachycardia, hypotension, hypokalemia and encephalopathy. This clinical picture is reminiscent of theophylline/methylxanthine toxicity which presents with a similar toxidrome by a more indirect pathway. Clenbuterol is a non-selective beta-adrenergic agonist that is seen more commonly today as an illicit drug adulterant or as a means for fat-burning in the body-building realm.
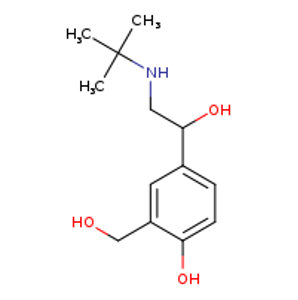
The toxidrome seen in this case can be explained through careful consideration of the chemical structure and the receptors affected. Structurally, clenbuterol is similar to salbutamol and our biogenic amines (e.g. epinephrine and norepinephrine). The tachydysrhythmia can be directly connected to the beta-1 agonist effects on the heart leading to increased inotropy, chronotropy and dromotropy. The hypotension can be explained by the beta-2 agonism on the smooth vasculature (this generates intracellular cAMP which inhibits the myosin-kinase light chain resulting in smooth muscle relaxation). The profound hypokalemia seen is similar to the effect of treating hyperkalemia with albuterol: the potassium is shifted intracellularly via the beta-2 induced Na+/K+ ATPase on skeletal muscle. Finally, the anion gap acidosis can likely be attributed to the vestigial beta-3 receptor on the mitochondria that uncouples oxidative phosphorylation (similar to effects seen with dinitrophenol or salicylate toxicity) leading to lactic acidosis and thermogenesis.
Traditionally, beta-agonist toxicity can be treated with esmolol, a cardioselective beta-blocker with rapid onset. The greatest limitation in the case of clenbuterol toxicity is the very short duration of action of esmolol. Alternative beta-blockers, such as labetalol, have limited utility given the potential to worsen hypotension through alpha1-antagonism. Typically, an alpha-agonist, such as phenylephrine or norepinephrine is necessary to maintain hemodynamic stability. In this case, symptoms persisted and were refractory to a maximal dose of esmolol and phenylephrine support, thus addition of a longer-acting, non-selective beta-blocker (propranolol) was recommended. Propranolol not only treated many of the symptoms, but also provided a duration of action that allowed for downward titration of esmolol and, ultimately, discontinuation of all parenteral vasoactive agents. An added benefit to propranolol is its high lipophilic nature which aids in a drug’s ability to cross the blood-brain barrier and achieve effective neuropenetration; specifically, in this case, it seemed to provide the patient with almost immediate anxiolytic effects. Furthermore, transitioning a patient to enteral therapy almost always provides a cost savings, particularly when considering the cost of pre-made esmolol infusions and the need for ICU-level of care. Propranolol provided safe and effective adjunctive therapy in this case of significant clenbuterol toxicity.
References
- Hoffman RJ, Hoffman RS, Freyberg CL, et al. Clenbuterol ingestion causing prolonged tachycardia, hypokalemia, and hypophosphatemia with confirmation by quantitative levels. Clin Toxicol 2001;39(4):339-344.
- Rose BD, Post TW. Clinical physiology of acid-base and electrolyte disorders. McGraw-Hill; New York: 2001.
Clenbuterol
Epinephrine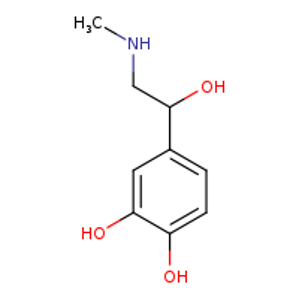
Salbutamol